
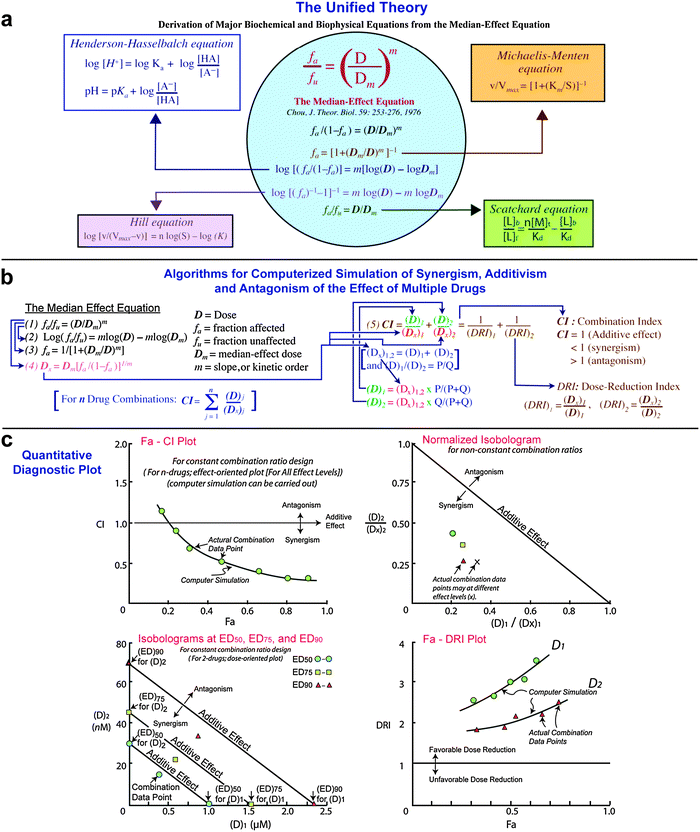
9 We found that the transcript levels of all CK2 subunits (α, α′ and β) were significantly higher in patient T-ALL cells, compared to normal T cells regardless of their developmental stages ( Figure 1A and B and Online Supplementary Figure S1A). To address this question, we analyzed publicly available databases and cross-compared the expression of CK2 subunits among subsets of developing T cells and patient T-ALL cells that are arrested at different developmental stages. Here we show that CK2 inhibition by CX-4945 destabilizes NOTCH1 and synergizes with JQ1 to induce apoptosis in human T-ALL cells, implicating an alternative strategy to target NOTCH1 signaling in refractory/relapsed T-ALL.ĬK2 (α and β) was previously found up-regulated in human T-ALL cells 8 whether this upregulation is linked to the temporal regulation of CK2 during T-cell development is unknown. 7 However, it remains unclear whether the cytotoxic effect of CX-4945 on T-ALL cells is associated with repression of NOTCH1 signaling. 5 CK2 inhibition by CX-4945, a potent and specific inhibitor in clinical trials for treating breast cancer and multiple myeloma, significantly reduces growth and survival of human T-ALL cells, 6 and down-regulates NOTCH1 in lung cancer cells. Protein kinase CK2 is a tetrameric serine-threonine kinase composed of two catalytic (α or α′) and regulatory (β) subunits that can phosphorylate NOTCH1. Identification of drug(s) synergizing with JQ1 to kill T-ALL cells may enhance the efficacy while reducing toxicities. 4 However, global repression of transcription is predicted to cause toxicities. 3 Targeting MYC-mediated transcriptional programs through BET bromodomain inhibitor JQ1 exhibits anti-leukemic efficacy in vitro and in vivo.
#Calcusyn software driver
3 The proto-oncogene MYC is a transcriptional target of NOTCH1 and a dominant driver of T-ALL pathogenesis.
The suppression of aberrant NOTCH1 signaling in T-ALL cells by gamma secretase inhibitors (GSIs) has been met with much enthusiasm however, the gastrointestinal toxicities and drug resistance of GSIs restrain their clinical applications. 2 1 Frequent application of multi-agent cytotoxic drugs leads to disease relapse and high toxicities, underscoring the need for targeted therapies. T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive cancer of developing thymocytes, and remains fatal in 20% of pediatric and 50% of adult patients.


 0 kommentar(er)
0 kommentar(er)
